Introduction to Various Battery Chemistries
Why Different Battery Types Exist
Numerous battery types have been created in the field of electrochemical energy storage. The differing demands across various applications are what led to the development of these unique battery chemistries. In terms of energy density, power density, cycle life, safety, temperature sensitivity, cost, and environmental effect, many applications have specific requirements. A pacemaker battery, for instance, would need to be extremely dependable and have a long lifespan, but an electric car battery would need to have great energy and power density. Therefore, none of the battery chemistry is suitable for all applications, many battery types have been created, each with a unique combination of properties and trade-offs.
Common Applications For Each Battery Type
Lead-Acid Batteries: They have been in use for more than a century and are renowned for being dependable and affordable. They are useful for situations where weight is not a deciding issue because of their low energy density and weight. Backup power supply (UPS), automotive starting batteries, and renewable energy storage are typical uses.
Nickel-Metal Hydride (NiMH) Batteries: In comparison to nickel-cadmium batteries, these batteries have a higher energy density and are more ecologically friendly. They are frequently found in rechargeable AA and AAA batteries, certain older-model electric cars, and hybrid electric vehicles.
Lithium-ion (Li-ion) Batteries: Lithium-ion batteries have established themselves as the industry standard for portable devices like smartphones and laptops. They are also widely utilized in electric cars due to their high energy density, low weight, and flexibility.
Lithium Iron Phosphate (LiFePO4) Batteries: LiFePO4 batteries, a subtype of lithium-ion batteries, are safer because they have a longer cycle life and are more thermally stable. They are utilized in applications including electric automobiles, power equipment, and large-scale energy storage where safety and cycle life are crucial.
Nickel-Cadmium (NiCd) Batteries: These batteries have a long cycle life and are noted for being able to produce large currents. But because they include cadmium, they have a memory effect and are less ecologically friendly. They are utilized in devices like two-way radios and power equipment.
Solid-State Batteries: Solid-state batteries, an emerging technology, employ a solid electrolyte rather than a liquid or polymer as the electrolyte. This may offer increased energy densities and enhanced safety. Despite being in the development phase, they are a viable choice for electric cars and portable gadgets.
Flow Batteries: These batteries have a large capacity and a long cycle life because they store energy in liquid electrolytes. Particularly for grid-scale energy storage applications, they are well suited.
Zinc-air Batteries: They are reasonably priced and have a high energy density. They are frequently employed in hearing aids, but they are also being investigated for application in grid storage and electric cars.
In order to satisfy the unique requirements of various applications, several battery chemistries are developed. Selecting the right battery for a particular application requires an understanding of the underlying chemistry and properties of each battery type.
The image below shows how we might arrange the various battery kinds according to their energy densities:
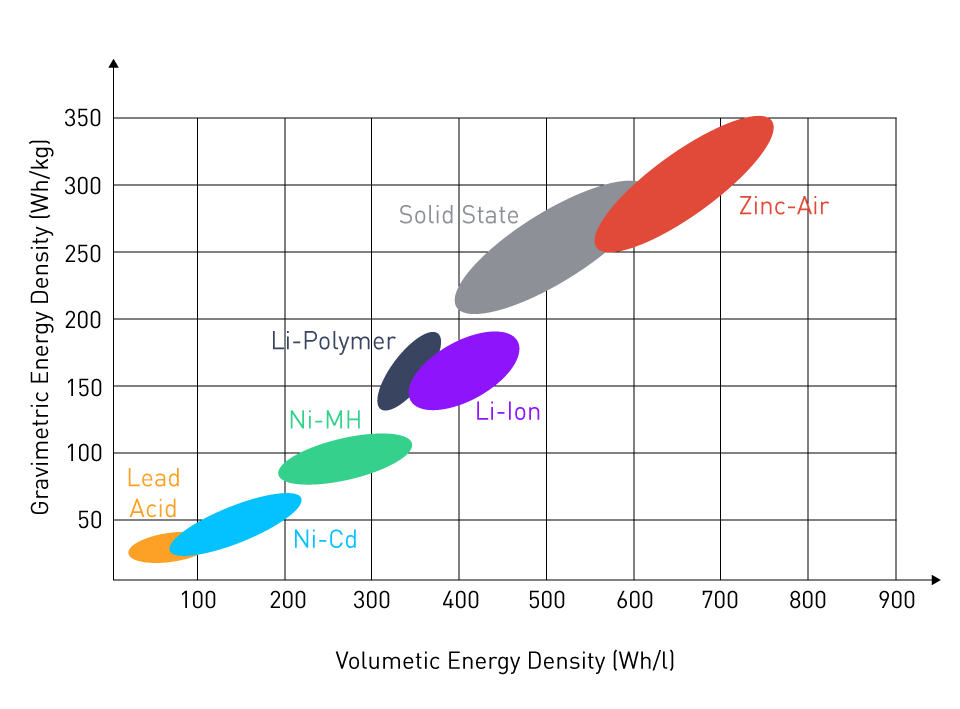
Figure 4: Graviometric and Volumetric Energy Density Comparison of Batteries
Lead-Acid Batteries
Chemistry And Operation
One of the first types of rechargeable batteries to be developed was the lead-acid battery, and since that time, in the middle of the 19th century, nothing has changed in terms of the chemistry that underlies lead-acid batteries' basic operation. Lead dioxide serves as the positive electrode of a lead-acid battery, while sponge lead serves as the negative electrode, and an electrolyte (solution of sulfuric acid) makes up the battery's essential elements.
Lead sulfate and water are produced by the reaction of the sulfuric acid electrolyte with the lead dioxide (positive plate) and sponge lead (negative plate) when the battery is discharged. By way of this reaction, an electric current is produced that may be used to power outside objects.
During discharge: PbO2 + Pb + 2H2SO4 -> 2PbSO4 + 2H2O + energy
At the point of time when the battery is recharged, an external voltage is provided, and the lead sulfate is transformed back into lead dioxide and sponge lead while the sulfuric acid is recycled.
During charging: 2PbSO4 + 2H2O + energy -> PbO2 + Pb + 2H2SO4
This reversible chemical reaction that lead-acid batteries use, enables them to undergo several cycles of charging and discharging. But when compared to more modern battery technologies, lead-acid batteries are less effective and have a lower energy-to-weight ratio. If discharged for a lengthy period of time, they are also prone to sulfation, which can reduce their lifespan and performance.
Applications: Automobiles, UPS, etc.
Lead-acid batteries are useful in a multitude of applications despite their shortcomings because of their dependability, well-established production techniques, and affordability.
Automobiles: These batteries are most frequently used as starting batteries for internal combustion engine automobiles. They are used to crank the engine by supplying a large current for a brief period of time. The battery is recharged by the vehicle's alternator once the engine is operating.
Uninterruptible Power Supplies (UPS): Lead-acid batteries are utilized to supply backup power in case of a main power loss in data centers and critical infrastructure. They are prized in this position because of their capacity to deliver great power for a short period of time.
Renewable Energy Storage: Energy storage in both off-grid and grid-connected renewable energy sources uses lead-acid batteries. Large quantities of energy from sources like solar or wind turbines may be stored by them, then released as needed.
Electric Wheelchairs and Golf Carts: Lead-acid batteries are frequently used in electric wheelchairs and golf carts due to their capacity to produce high current and comparatively inexpensive price.
Emergency Lighting and Alarm Systems: In emergency lighting and alarm systems, when dependable performance is vital, lead-acid batteries are employed.
Nickel-Metal Hydride (NiMH) Batteries
Chemistry And Operation
Rechargeable Nickel-Metal Hydride (NiMH) batteries are widely used in the consumer electronics industry and more recently in automotive applications. The chemistry of a NiMH battery consists of an alkaline electrolyte, often potassium hydroxide (KOH), a negative electrode that is normally produced from a metal hydride (commonly alloys of lanthanum, cerium, neodymium, and other rare earth metals), and a positive electrode composed of nickel hydroxide (NiOOH).
The following are the primary electrochemical processes that take place throughout the charging and discharging operations:
During Charging:
- Positive electrode (NiOOH + H2O + e- -> Ni(OH)2 + OH-)
- Negative electrode (MH + OH- -> M + H2O + e-)
During Discharging:
- Positive electrode (Ni(OH)2 + OH- -> NiOOH + H2O + e-)
- Negative electrode (M + H2O -> MH + OH-)
These reversible reactions recharge the batteries multiple times. NiMH batteries have higher energy density than their predecessor, Nickel-Cadmium (NiCd) batteries. In addition to this, as compared to the NiCd batteries, these NiMH batteries do not suffer from the “memory effect” that means they can maintain their capacity without requiring complete discharge cycles.
Applications: Hybrid Cars, Rechargeable AA/AAA, etc.
Hybrid Cars: In the automobile sector, NiMH batteries are frequently used, particularly in hybrid electric vehicles (HEVs). They are great for the power needs of hybrid vehicles since they provide a good balance between performance, energy density, and affordability. For the harsh automotive environment, its toughness and capacity to withstand heavy charge and discharge cycles are advantageous. NiMH batteries are thought to be significantly safer than Li-ion batteries, which is also a big plus in HEV automobiles. However, these are less approached vehicles as compared to EV because of their lower energy density.
Rechargeable AA/AAA Batteries: NiMH batteries have established themselves as the industry standard for rechargeable AA and AAA batteries in consumer electronics. They are more cost-effective and environmentally friendly than single-use alkaline batteries because of their high energy density and numerous rechargeable capabilities.
Power Tools: In cordless power tools where a large current draw is necessary, NiMH batteries are employed. They can provide the necessary power for motors and can be recharged, so there is less of a need to replace them frequently.
Emergency Lighting Systems: NiMH batteries are a popular option for emergency lighting systems when continuous preparedness and performance are crucial due to their dependability and rechargeability.
Lithium-ion (Li-ion) Batteries
Chemistry and Operation
Rechargeable lithium-ion batteries, sometimes known as Li-ion batteries, are the technology of choice for a variety of applications, including consumer electronics, electric cars, and grid storage. When compared to other rechargeable batteries, this is mostly because of their high energy density, low self-discharge, and great cycle ability.
The flow of lithium ions between the positive and negative electrodes constitutes the fundamental chemistry of a Li-ion battery. Lithium cobalt oxide (LiCoO2) or closely related substances like lithium manganese oxide (LiMn2O4) or lithium iron phosphate are typically used to make the positive electrode (cathode). Graphite is frequently used as the negative electrode (anode). Apparently, a lithium salt in an organic solvent serves as the electrolyte.
The following electrochemical processes occur while charging and discharging:
During Charging:
- Cathode: LiCoO2 -> Li(1-x)CoO2 + xLi+ + xe-
- Anode: xLi+ + xe- + 6C -> LixC6
During Discharging:
- Cathode: Li(1-x)CoO2 + xLi+ + xe- -> LiCoO2
- Anode: LixC6 -> xLi+ + xe- + 6C
Lithium ions are transferred from the positive electrode to the negative electrode during the charging process. The ions return to the positive electrode during discharge.
Careful control of the charging and discharging cycles is essential when using Li-ion batteries to avoid overcharging or deep draining, which can make the battery unstable and potentially harmful.
Applications: Smartphones, Laptops, Electric Vehicles, etc.
Smartphones and Portable Electronics: Smartphones and other portable electronics frequently use Li-ion batteries. They are perfect for applications where weight and compactness are important, and long battery life is sought. This is possible due to their high energy density and minimal self-discharge.
Laptops: Li-ion batteries are the preferred option for laptop computers, much like they are for smartphones. The batteries need to be small, light, and able to maintain a charge for a long time; Li-ion technology is ideal for these needs.
Electric Vehicles (EVs): Electric cars are one of the most innovative uses of Li-ion batteries. The high energy density of Li-ion batteries, which is essential for obtaining tolerable ranges on a single charge, has led the automobile industry to select them as the standard for EVs. However, the risk of thermal risks is heightened.
Energy Storage Systems (ESS): Li-ion batteries have been used in energy storage systems as a result of the rise in renewable energy sources. They are used to store surplus energy produced, for instance, by solar panels or wind turbines, so that it may be used at a later time when neither the sun nor the wind are present.
Power Tools and Drones: Li-ion batteries are preferred for high-performance applications like power tools and drones due to their capacity to discharge large currents as well as their lightweight and tiny size.
Lithium-Iron-Phosphate Batteries
Chemistry and Operation
Batteries that use lithium iron phosphate as the cathode are known as lithium-iron-phosphate (LiFePO4) batteries, sometimes known as LFP batteries. Due to its significant benefits over other lithium-ion chemistries, such as improved thermal stability, safety, and a longer cycle life, this chemistry is becoming more and more common.
The anode is commonly constructed of graphite, the cathode is lithium iron phosphate, and the electrolyte is a lithium salt in an organic solvent in LiFePO4 batteries. The following are the basic electrochemical reactions that occur during the charge and discharge processes:
During Charging:
- Cathode: FePO4 + Li+ + e- -> LiFePO4
- Anode: C + Li+ + e- -> LiC6
During Discharging:
- Cathode: LiFePO4 -> FePO4 + Li+ + e-
- Anode: LiC6 -> C + Li+ + e-
The absence of oxygen release, which may lead to thermal runaway in conventional lithium-ion chemistries, is one of the crucial characteristics of LiFePO4 batteries. This increases their degree of safety, especially when exposed to high temperatures or mechanical damage. It's important to note that this increased degree of safety is still not on par with that of lead-acid or nickel-metal hydride batteries.
LiFePO4 has a relatively flat discharge curve as well. As a result, the voltage maintains a consistent level throughout battery discharge, which is helpful in applications requiring a stable output voltage.
Applications: Energy storage, vehicles, UPS, etc.
Energy Storage Systems (ESS): For stationary energy storage systems, such as those used in combination with renewable energy sources like solar or wind power, LiFePO4 batteries are a good fit. They are perfect for this application because of their long cycle life, safety, and thermal stability.
Electric Vehicles (EVs): Due to its higher thermal stability and safety, LiFePO4 batteries are chosen by some electric car manufacturers over alternative lithium-ion chemistries. Despite having a somewhat lower energy density than other Li-ion chemistries, they can have a substantial benefit in electric cars due to their longer cycle life.
Uninterruptible Power Supplies (UPS): LiFePO4 batteries are a desirable alternative for UPS systems because of their safety and extended cycle life. UPS systems need great dependability to provide a consistent power supply in the case of a power loss.
Recreational Vehicles (RVs) and Marine Applications: LiFePO4 batteries are used in RVs and marine applications because they can withstand harsh working conditions and have great thermal stability.
Portable Power Packs and E-bikes: They are perfect for electric bicycles and portable power packs because of their dependability, safety, and lightweight design.
Other Battery Types
Nickel-Cadmium (NiCd)
NiCd or NiCad batteries, often known as nickel-cadmium batteries, are rechargeable batteries that use metallic cadmium and nickel oxide hydroxide as electrode materials. Cadmium (Cd) serves as the negative electrode, while nickel oxide hydroxide (NiOOH) serves as the positive electrode. Typically, potassium hydroxide (KOH) serves as the electrolyte. The following are the charge and discharge reactions in a NiCd battery:
During Charging:
- Positive electrode: Ni(OH)2 + OH- -> NiOOH + H2O + e-
- Negative electrode: Cd + 2OH- -> Cd(OH)2 + 2e-
During Discharging:
- Positive electrode: NiOOH + H2O + e- -> Ni(OH)2 + OH-
- Negative electrode: Cd(OH)2 + 2e- -> Cd + 2OH-
NiCd batteries can withstand a large number of charge-discharge cycles and have a long shelf life. However, due to the memory effect and the presence of hazardous cadmium, they have been mostly replaced by more ecologically friendly alternatives.
Solid-State Batteries
By using solid-state materials for both the electrolyte and the electrodes, solid-state batteries promise a revolutionary way to store energy. Ceramics, polymers, or a mix of the two can all be used to create the solid electrolyte. With this design, there is no need for flammable or leaky liquid or gel electrolytes. In comparison to conventional liquid-based lithium-ion batteries, solid-state batteries are thought to have the potential to provide better energy densities, enhanced safety, and longer cycle lifetimes. However, this technology still has a number of issues that need to be overcome. Currently, the cost of manufacture prevents large-scale production. Both the electrode-to-electrolyte interface resistance and the electrolyte resistance are greater in solid-state batteries. When charging and discharging activities occur, they frequently encounter cracks inside the solid electrolyte.
Flow Batteries
Redox flow batteries or Flow batteries are a special kind of rechargeable battery that store energy in liquid electrolyte solutions that are external to the battery cell. The ion exchange required for charging and discharging is facilitated by the pumping of electrolytes through electrodes within the cell that are physically isolated from one another by a membrane. The scaling of energy capacity and power output may be done separately thanks to this architecture. Flow batteries are especially well-suited for large-scale energy storage applications, such as grid storage, because of their scalability, high cycle life, and capacity to discharge energy for prolonged durations.
Zinc-air Batteries
Metal-air batteries called zinc-air batteries get their energy from oxidizing zinc with oxygen from the surrounding air. These batteries are an appealing alternative for different applications because of their high energy density and low weight. A zinc oxide, an electrolyte (often potassium hydroxide), and a cathode with a porous shape that allows air to seep in are the essential components. When zinc is discharged, it oxidizes to produce zinc oxide and releases energy in the process. Zinc-air batteries are frequently used in hearing aids due to their high energy density and low cost, and they are also being investigated for larger-scale applications including electric vehicles and grid storage.






直接登录
创建新帐号