Introduction
An electrochemical device known as a battery transforms chemical energy into electrical energy through redox processes, or we may do the opposite and transform electrical energy into chemical energy. It is made up of one or more electrochemical cells, each with an electrolyte, two electrodes (the anode and the cathode), and other components. The cathode is the positive electrode and is the location of reduction, whereas the anode is the negative electrode and is where oxidation occurs. The passage of electric current via the electrodes' external circuit is made possible by the electrolyte, which makes it easier for ions to transfer between the electrodes. Batteries can be classified as primary or secondary. Primary batteries are disposed of after use and cannot be refilled. The essential elements of a battery cell are shown in the following image. As we can see, the cell's anode and cathode terminals exhibit useful voltage.
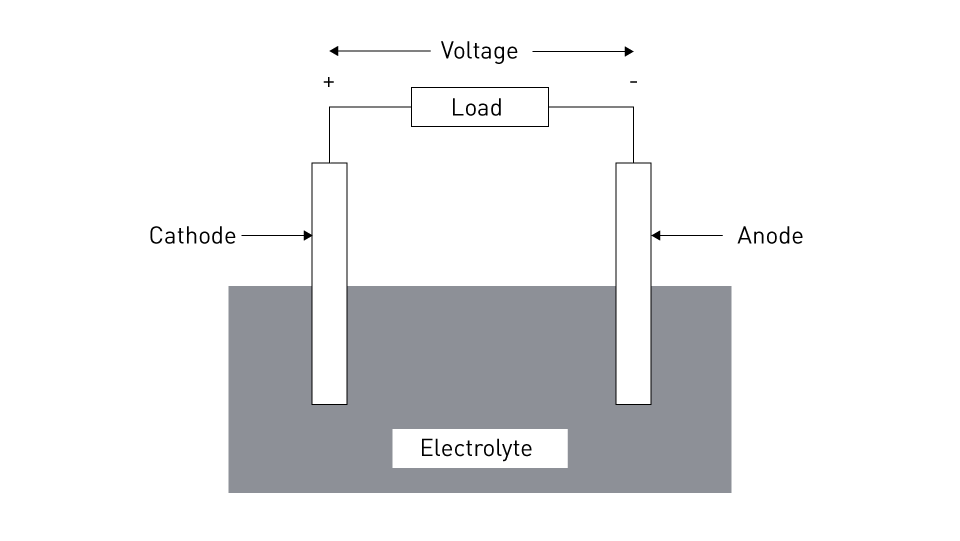
Figure 1: Components of a Cell
Batteries play a crucial function in the contemporary world. The technology we use and the way we live have changed dramatically as a result of their mobility and energy storage capabilities. Batteries have made important contributions in the following areas:
Mobility and Communication: A wide variety of portable gadgets, including cell phones, laptops, and tablets, depend on batteries for communication and information access.
Transportation: Batteries are at the forefront of the sustainable transportation movement because of the introduction of electric cars (EVs). They make it possible for cars to operate without directly utilizing fossil fuels, hence lowering pollution and greenhouse gas emissions.
Renewable Energy Storage: The integration of renewable energy sources like solar and wind power depends heavily on batteries. They increase the dependability and effectiveness of renewable energy systems by storing energy produced during times of high output for use during times of low production or peak demand.
Medical Applications: Batteries are essential in many medical devices, including hearing aids, pacemakers, and portable medical equipment. They enable flexibility and independence in the care and treatment of patients.
Emergency Preparedness and Response: In times of power outages and natural calamities, batteries offer a crucial energy source. They provide energy for radios, flashlights, and other life-saving emergency supplies.
Consumer Electronics: Batteries power commonplace goods like remote controls, cameras, and toys, making them portable and convenient.
Ancient Batteries: The Baghdad Battery
A group of curious items was unearthed in the 1930s not far from the Khuyut Rabbou'a, close to Baghdad, Iraq. Some people think that these objects, which are now known as the Baghdad Battery, date back to roughly 200 BCE and are the first known instances of batteries. A copper cylinder is contained inside each Baghdad Battery, which is made of a clay jar that is 13 cm tall, and is secured in place by bitumen (asphalt). An oxidized iron rod is housed inside of this copper container.
The structure remarkably resembles a simple galvanic cell. An electrolyte, such as lemon juice or vinegar, can be added to the jar. When the jar is filled with an electrolytic solution, the copper and iron serve as electrodes, and it is assumed that an electric current will be produced.
Possible Uses and Speculations
Historians and archaeologists continue to speculate about and disagree over the Baghdad Battery's true purpose. Here are some hypotheses:
Electroplating: According to some academics, these antique batteries may have been used to electroplate things with gold or silver. Metal can be deposited onto an object, such as a piece of jewelry, by passing a current through a solution containing metal ions. The electroplating procedure might have been utilized for ceremonial or religious purposes.
Pain Relief: According to a different notion, the Baghdad Battery was employed for medical treatment, notably electrotherapy for pain management. Electric fish were used by the ancient Greeks to treat pain, and it's probable that Mesopotamians also discovered a means to achieve a similar result with the help of these primitive cells.
Religious Rituals: According to some conjecture, the batteries may have been utilized in religious ceremonies. It was possible for a weak current to be linked to an item and generate a sensation or movement that may be seen as having mystical or religious overtones.
Simple Curiosity or Experimentation: It's also conceivable that the Baghdad Battery was developed without any particular use in mind out of pure curiosity and experimenting with the characteristics of various materials.
Birth of Modern Batteries
Alessandro Volta and The Voltaic Pile (1800)
Modern batteries were created around the turn of the 19th century. The first real battery was created in 1800 by an Italian physicist by the name of Alessandro Volta. This device is now referred to as the voltaic pile. Luigi Galvani's research, which showed that a frog's leg would twitch when two different metals (such as copper and zinc) were linked and touched, served as the inspiration for Volta's discovery. This, according to Galvani, was brought on by the "animal electricity" present in the frog. Volta disagreed, explaining the twitching as the result of an electrical current moving between the two metals.
Volta set out to demonstrate his idea by developing a machine that could generate a constant flow of electricity. He built an electrolyte out of brine-soaked fabric or cardboard placed between layers of zinc and copper. Electricity flowed via a wire linked from the top to the bottom of the stack because the two materials had differing electronegativities that required electrons to go from zinc to copper, generating the flow of electric current. The voltaic pile, a ground-breaking innovation, served as the first reliable source of electric current and is regarded as the precursor of contemporary batteries. Alessandro Volta made significant contributions to science, and his name was given to the unit of electric potential, the Volt.
Daniell Cell (1836)
The voltaic pile was a revolutionary invention, but it had drawbacks. The most notable of them was a voltage drop with prolonged usage, principally brought on by the accumulation of hydrogen bubbles on the copper electrodes. The English scientist John Frederic Daniell addressed this phenomena, also known as polarization, in 1836.
Daniell created the Daniell cell, a brand-new kind of battery. In his invention, an unglazed earthenware container was submerged in a copper pot that contained a solution of copper sulfate. A zinc electrode and sulfuric acid were placed into the pottery. While allowing ions to get through, the porous earthenware prevented the two solutions from immediately combining.
This arrangement provided a more stable and constant voltage than the voltaic pile while successfully preventing the accumulation of hydrogen bubbles on the copper electrode.
The Daniell cell marked a significant development in battery technology and was adopted widely to power early telegraph and telephone networks. It was one of the earliest examples of clever engineering being used to meet and overcome the constraints of existing technology. Volta and Daniell's combined efforts set the groundwork for the creation of contemporary batteries.
Twentieth Century Developments
Introduction of Lead-Acid Batteries
The French physicist Gaston Planté created the lead-acid battery in 1859, and it is a significant invention that gained real recognition in the 20th century. It turned into the first rechargeable battery to be utilized in industrial settings. The lead-acid battery produces a lot of current quickly by using lead dioxide as the positive plate, sponge lead as the negative plate, and sulfuric acid as the electrolyte. It became the battery of choice for car starting motors due to its capacity to deliver large surge currents and economical manufacturing. The lead-acid battery continued to advance during the 20th century with improvements like the sealed lead-acid battery, which requires no maintenance and can be used in any orientation.
The Advent of Alkaline Batteries
The introduction of the alkaline battery was another important breakthrough that occurred in the 1950s. The alkaline battery was created by Canadian engineer Lewis Urry when he was employed at the Eveready Battery business as a replacement for zinc-carbon batteries. Zinc and manganese dioxide are used as the electrodes in alkaline batteries, together with an alkaline electrolyte, often potassium hydroxide. Compared to zinc-carbon batteries, the alkaline electrolyte improves the energy density and shelf life. Due to its extended lifespan, alkaline batteries soon became popular as main batteries in consumer electronics including toys, flashlights, and portable gadgets where rechargeable batteries are not necessary.
Nickel-Cadmium Batteries
The need for rechargeable batteries with better energy densities became clear as the demand for portable electronic gadgets increased. Waldemar Jungner created the Nickel-Cadmium (NiCd) battery in 1899, and it was improved upon in the early to mid-20th century. This battery type has an alkaline electrolyte and electrodes made of nickel oxide hydroxide and cadmium. One of the earliest types of rechargeable batteries utilized in consumer devices were nickel-cadmium batteries. Nevertheless, despite their widespread usage, cadmium, a hazardous heavy metal, caused environmental issues. As a result, nickel-metal hydride batteries (NiMH), which are similar to cadmium batteries but employ a hydrogen-absorbing alloy instead, were created in the latter part of the 20th century.
In the development of battery technology, the 20th century marked a turning point. The development of lead-acid, alkaline, and nickel-cadmium batteries enabled a variety of uses, from cars to portable gadgets, and laid the groundwork for the current era of battery technology.
Twenty-First Century and Beyond
Lithium-ion Batteries
With the widespread acceptance and advancement of lithium-ion batteries, the turn of the twenty-first century saw a tremendous change in battery technology. Despite the fact that lithium-ion batteries were created in the 1980s, it wasn't until the 2000s that they were widely accepted for use in portable gadgets, electric cars, and renewable energy storage systems.
Higher energy densities, lower self-discharge rates, and the absence of the memory effect—a problem with nickel-cadmium batteries—are just a few advantages that lithium-ion batteries have over earlier technologies. With nickel-cadmium batteries, if they were only partially discharged before being recharged too frequently, their overall capacity was reduced, as if they were remembering the previous discharge state. A lithium cobalt oxide cathode, a graphite anode, and a lithium salt in an organic solvent serve as the components of a lithium-ion battery. Other chemistries have also been created throughout time, each with its own distinct set of properties, such as lithium iron phosphate (LiFePO4) and lithium nickel manganese cobalt oxide (LiNiMnCoO2).
The awarding of the 2019 Nobel Prize in Chemistry to John B. Goodenough, M. Stanley Whittingham, and Akira Yoshino for the creation of lithium-ion batteries was one of the defining events of the twenty-first century in terms of lithium-ion technology.
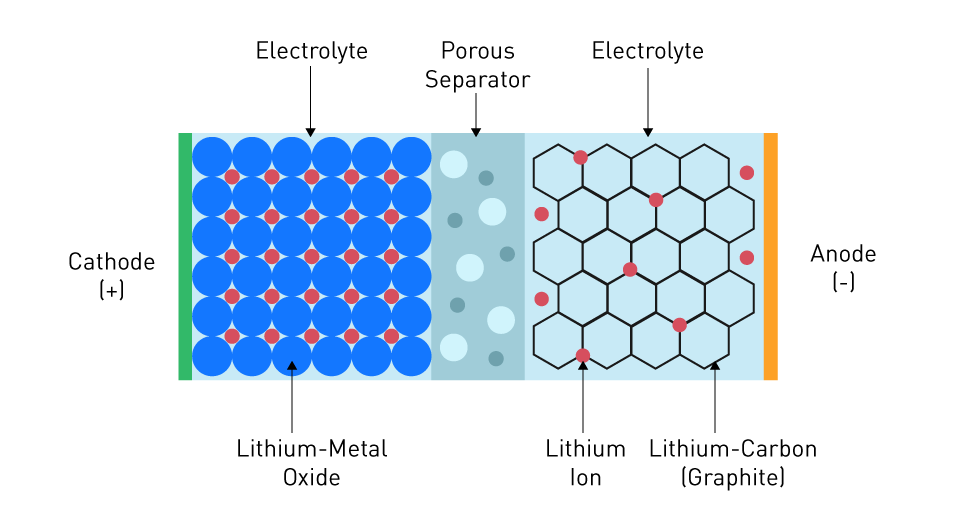
Figure 2: Structure of a Lithium-Ion battery
Ongoing Research And Future Technologies
The need for batteries with increasingly better energy densities, quicker charging periods, and improved safety is only increasing as the twenty-first century goes on. Several active research projects and cutting-edge technologies hold the potential to completely transform the battery industry.
Solid-State Batteries: The creation of solid-state batteries is one of the most anticipated technological advances. These batteries use a solid electrolyte rather than a liquid one, which may result in better energy densities, quicker charging, and improved safety. Although solid electrolytes were initially identified in the 19th century, a number of issues have hindered their widespread use. Innovations in the late 20th and early 21st centuries , starting in the 2010s, have rekindled interest in solid-state battery technology. The commercialization of solid-state batteries is a top priority for businesses and researchers worldwide, particularly for applications involving electric vehicles.
Silicon Anodes: Research is now being done to replace the graphite anode in lithium-ion batteries with silicon. Because silicon can store a lot more lithium ions than graphite, the battery's energy density may rise. Problems with silicon's expansion and contraction during charging and discharging cycles are being solved, though.
Alternative Chemistries: Alternative battery chemistries including lithium-sulfur, sodium-ion, and magnesium-ion batteries are also the subject of research. These substitutes are designed to get around some of the drawbacks of lithium-ion technology or lessen reliance on components like cobalt that have limited supply chains.
Energy Storage Systems: A significant amount of research is being done on advanced energy storage systems that use renewable energy sources in addition to developments in battery technology. As different battery technologies have distinct unique properties, such as energy density, power density, cycle capabilities, and cost, these systems, which frequently combine numerous battery technologies, are vital for the worldwide transition to sustainable energy.
AI and Machine Learning for Battery Development: Another new advancement is the use of machine learning and artificial intelligence to speed up the development of batteries. These technologies can optimize battery designs, boost battery management systems, and enhance production procedures. By evaluating real-time data from sensors and production lines, AI can aid in the optimization of battery manufacturing processes. To find patterns and links between battery materials, components, and performance, AI systems can evaluate vast amounts of data. Advanced battery management systems that optimize charging and discharging tactics depending on operational conditions in real-time may be created using AI. AI can help with virtual battery testing and modeling, which can eliminate the need for elaborate physical prototypes.
Battery technology is expected to undergo extraordinary progress in the twenty-first century. The stakes have never been higher due to the threats posed by climate change and the switch to renewable energy sources. To effectively address the world's energy concerns, battery technology innovation and research must continue.






直接登录
创建新帐号