Introduction to Battery Parameters
Why Battery Parameters are Important
Batteries are an essential part of energy storage and delivery systems in engineering and technological applications. Understanding and analyzing the variables that define a battery's behavior and performance is essential to ensuring that batteries operate dependably and effectively in these applications. These criteria are essential for a number of reasons:
Selection and Sizing: Engineers can select the best battery for a certain application by knowing the parameters and calculating the size and number of batteries required to match the specifications.
Optimization: Engineers may increase battery life, efficiency, and safety by optimizing the system by knowing how a battery behaves under various situations, such as temperature ranges and charge-discharge cycles.
State Monitoring: The status of the battery may be determined by continuous monitoring of specific metrics, which is crucial for estimating the battery’s performance and remaining life.
Safety and Reliability: If batteries are not utilized within their acceptable working parameters, they might be harmful. The battery can run safely and dependably if the parameters are understood and monitored. For instance, keeping an eye on the temperature of the battery.
Economic Feasibility: When batteries are a large component of the system, as they are in electric vehicles or grid storage, they frequently play a crucial role in the economic viability of projects. We can consider, for instance, governmental regulations, maintenance costs, battery lifetime and disposal options, large-scale manufacturing options, price drops, etc.
Commonly used Parameters in Industry
Capacity: The entire energy in a battery is measured here, and it is usually expressed in ampere-hours (Ah). It provides information on how much charge the battery can deliver at a particular discharge rate.
Energy Density and Power Density: The quantity of energy stored per unit of mass or volume is measured by the energy density (Wh/kg or Wh/L). How much power can be delivered per unit of mass or volume is indicated by the power density (W/kg or W/L). In particular, these factors are crucial for portable and mobile apps.
State of Charge (SOC): This displays the battery's current charge level as a percentage of its capacity. It's a crucial variable for determining how much energy is still there in the battery.
State of Health (SOH): SOH is a measurement that depicts a battery's overall health and how long it has left to live in comparison to a brand-new battery. It considers elements including the number of cycles, capacity fading, and changes in internal resistance.
Nominal Voltage: It is the typical voltage at which the battery functions while charged and when subjected to typical operating circumstances.
Internal Resistance: The amount of energy lost as heat during operation depends on this characteristic, which is essential. Increased energy loss caused by a high internal resistance might potentially cause heating and safety problems.
Cycle Life: This indicates how many full charge/discharge cycles a battery may experience before its capacity drops below a specific percentage of its initial capacity.
C-rate: It shows how quickly a battery is losing capacity in relation to its maximum. A 1C rate indicates that the battery will be completely discharged in an hour by the discharge current.
Anyone working with battery systems, whether for design, maintenance, or analytical purposes, has to understand and handle these factors effectively. The secret to improving performance and prolonging the lifespan of battery systems may lie in understanding how these variables interact and vary over time.
Capacity
Definition and Units
The term "capacity," which is used to refer to a battery's ability to hold and distribute electrical charge, is indicated by the letter "C". It is a key variable that determines how much power a battery can deliver. The ampere-hour (Ah), which measures how much electric current a battery can produce for an hour, is the common unit of capacity. We determine the size of electrical charges by dividing the electrical current by the passing of time. The milliampere-hour (mAh), where 1 Ah = 1000 mAh, is a more useful measurement that is occasionally used, particularly for tiny batteries. The energy capacity is calculated in watt-hours (Wh) by multiplying the capacity (Ah) by the average voltage (V) during discharge.
Factors affecting Capacity
The capacity of a battery is affected by numerous factors:
Chemistry and Design: The composition and design of the battery's electrodes and electrolyte have a big impact on how much power it can store. A lithium-ion battery, for instance, often has a larger capacity than a lead-acid or nickel-metal hydride battery of the same size.
Temperature: A battery's capacity is temperature-dependent. Higher temperatures often cause rapid aging at the price of momentary capacity increases. As a result of the slower electrochemical processes, lower temperatures increase capacity.
Discharge Rate: The battery's capacity is impacted by the rate at which electricity is extracted from it. The available capacity declines as the discharge rate rises, a phenomenon known as the Peukert effect. Batteries are categorized according to the multipliers of capacity that define their maximum permitted discharge rate. Therefore, if a 10Ah battery's permitted discharge rate is 2C, the battery may only be drained with a maximum of 20 Amper.
Aging: The capacity of batteries decreases over time. This is brought on by a number of things, such as internal resistance growth, electrolyte loss, and active material deterioration.
State of Charge (SOC) and Depth of Discharge (DOD): The SOC and DOD of a battery also have an impact on its usable capacity. Over time, frequent deep discharges may cause the total capacity to decline.
Charge Method: A battery's capacity may be impacted by the method and rate of charging. For best capacity retention, some batteries require specialized charging strategies. Using the charge curve of a Li-ion battery as an example:
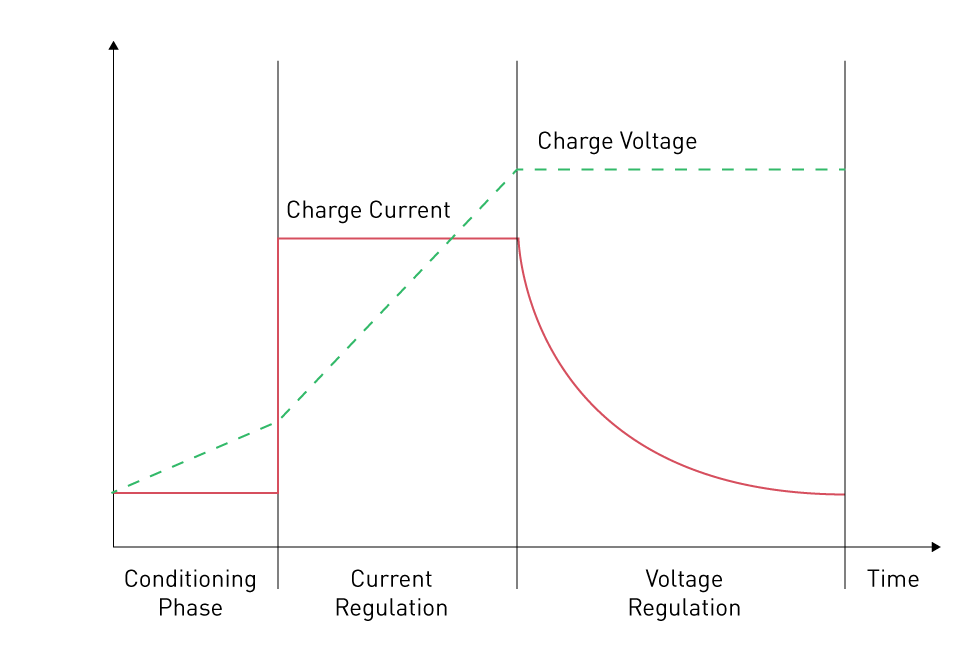
Figure 5: Typical Li-ion charge curve
To avoid damaging or aging the battery while it is deeply depleted, we must charge it in accordance with the needs of the Conditioning Phase. This implies that until the battery voltage increases over the deep-discharge level, a continual low quantity of current must be employed. The current regulation phase begins when the battery voltage reaches a certain level. We can use the maximum charging current permitted during this phase to charge the Li-ion battery. We enter the Voltage Regulation phase when the battery is operating at its maximum level, which for Li-ion cells is normally between 4.1V and 4.2V. We must charge the battery with a consistent voltage throughout this phase. The charging current rapidly decreases when the battery voltage hits its maximum permitted voltage until it reaches a very low level, often below 50mA, which is regarded as the conclusion of the charging operation.
Typical Values for Different Battery Types
Lead-Acid Batteries: Small lead-acid batteries typically have a capacity of approximately 1 Ah, whereas huge deep-cycle batteries used in renewable energy systems have a capacity of over 200 Ah.
Nickel-Metal Hydride (NiMH) Batteries: For AA and AAA sizes, these batteries generally have capacities between 600 mAh and 2.5 Ah. The capacity of larger NiMH batteries used in electric cars can exceed 100 Ah.
Lithium-ion (Li-ion) Batteries: The capacity of a common Li-ion cell in the 18650 size ranges from 1.5 Ah to 3.5 Ah. Electric car batteries with larger pouch or prismatic cells can have capacities ranging from 20 Ah to more than 200 Ah.
Lithium Iron Phosphate (LiFePO4) Batteries: The capacity of these batteries are often a little bit lower than those of other lithium-ion chemistries. Depending on size and application, cells typically vary from 15 Ah to 200 Ah.
Nickel-Cadmium (NiCd) Batteries: These batteries typically have a lesser capacity than NiMH batteries, with an average capacity of 600 mAh for AA-size batteries and 50 Ah for bigger cells.
Energy Density and Power Density
Definitions and Differences
Power Density and Energy Density are important factors to consider while describing and choosing batteries for various purposes. Let's define each and see how they vary from one another.
Energy Density: The energy density of a battery, which is sometimes represented by the letter "U," is a measurement of how much energy it can hold relative to its volume or mass. Gravimetric energy density (Wh/kg), which measures energy stored per unit of mass, and volumetric energy density (Wh/L), which measures energy stored per unit of volume, are the two ways it is generally stated. For a given size or weight, a battery with a higher energy density may store more energy, which is especially useful for portable applications.
Power Density: Power density, which is sometimes represented by the letter "P," is a measurement of how rapidly a battery can supply energy. Similar to energy density, it may be stated in two different ways: volumetric power density (W/L), which represents power delivery per unit volume, and gravimetric power density (W/kg), which represents power delivery per unit mass. Applications that call for brief bursts of energy can benefit from the high power output that a battery with a high power density can deliver for a given size or weight.
The nature of the characteristics that each density represents is where the main distinction between energy density and power density exists. Power density is concerned with the pace at which energy can be delivered, whereas energy density is concerned with the total quantity of energy that can be stored.
Importance in Different Applications
Depending on the needs of various applications, energy density and power density have varying degrees of importance.
Electric Vehicles (EVs): Both energy density and power density are essential for electric cars. Energy density is crucial since it establishes a vehicle's range, or how far it can go on a single charge. Power density is also essential since it affects the vehicle's performance and acceleration.
Portable Electronics: Energy density is frequently more important than power density in portable electronics like smartphones and laptops. A portable device's ability to function for a longer period of time without recharging is made possible by having a high energy density.
Uninterruptible Power Supplies (UPS): Power density may be more important than energy density in UPS systems. This is because, in the event of a power outage, UPS systems must deliver a large quantity of power in a little amount of time.
Drones and Aerospace: Energy density and power density are both significant in drones and aeronautical applications. Long flight periods demand energy density, but lift and maneuverability require power density when a high mechanical torque is needed.
Grid Energy Storage: Energy density is frequently more crucial for grid energy storage systems because the main objective is to store huge amounts of energy. Power density becomes crucial in situations when a quick response to grid demands is necessary.
The following image shows the relationship between the energy density and power density of the most widely used batteries and other storage devices:
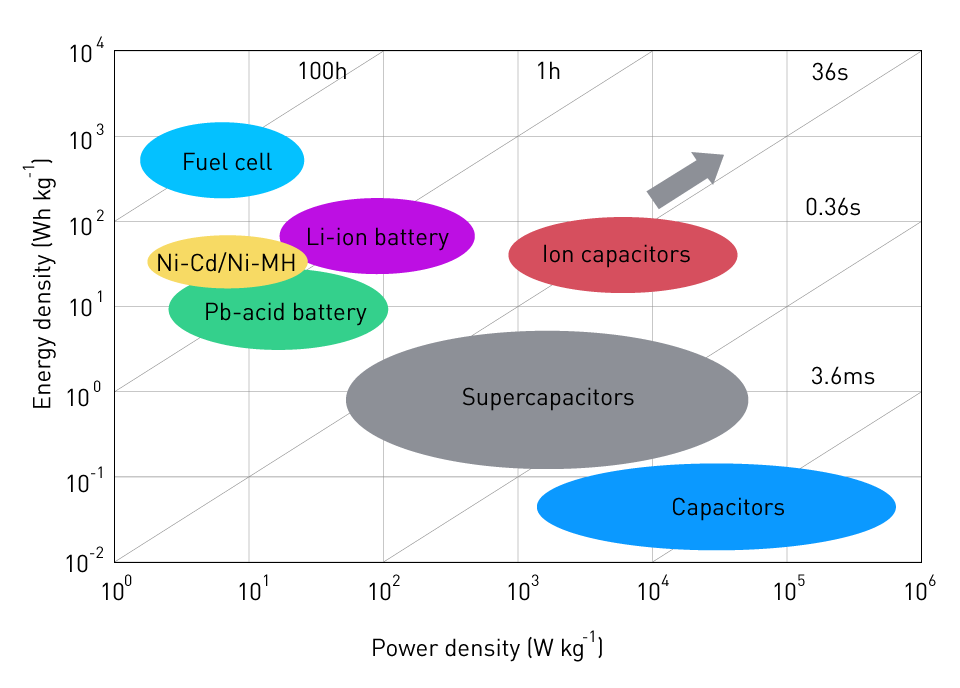
Figure 6: Energy and Power density of storage devices
As can be seen, Li-ion batteries have the highest power and energy densities of all the batteries. It's noteworthy to note that we're employing capacitors in this situation because of the high power density needs. Capacitors are limited in their ability to store energy but may release it quickly without losing any of their usefulness. In battery systems, capacitors are frequently included, with the battery just pre-charging the capacitor to have it ready for sudden load demands.
State Of Charge (SOC)
Definition and Importance
A crucial metric called "State of Charge" (SOC) shows how fully charged a battery is right now in relation to its capacity. It is often stated as a percentage, where 0% corresponds to a battery that is empty and 100% corresponds to a battery that is completely charged.
SOC is a vital data point since it gives users and battery management systems (BMS) important knowledge about how much energy is present in the battery. Knowing the SOC is important for the following reasons:
Operational Planning: Knowing the SOC can help with trip planning for electric vehicles, for example, to make sure that the destination can be reached without draining the battery.
Battery Health: A battery's lifespan can be shortened by repeatedly draining it to a very low SOC or charging it to a very high SOC. The SOC can direct charging procedures to enhance battery health.
Load Management: SOC may be utilized in energy storage systems to optimize energy expenditures by deciding when to charge or discharge the batteries based on power pricing.
Methods for Estimating SOC
Since a battery's internal chemical processes are not easily visible, estimating the level of charge of a battery is not simple. To estimate SOC, however, numerous techniques have been created:
Coulomb Counting: Observing the current entering and leaving the battery is required for this technique. The total accumulated or lost charges are calculated by gradually integrating this current. Calculating SOC can be done using this charge change in proportion to the battery's overall capacity. Coulomb counting may seem straightforward, but it suffers from cumulative mistakes over time as a result of efficiency losses and other factors, such as measurement tolerances.
Coulomb Counting + Open Circuit Voltage (OCV): With this technique, voltage readings at the battery terminals are combined with Coulomb counting. The open-circuit voltage of the battery and its charge level are connected. A more precise SOC estimation can be achieved by periodically measuring the OCV and using it to correct the accumulated mistake in the Coulomb counting approach. However, since many systems require a continuous energy supply from the battery, reliable OCV measurements necessitate the battery being at rest, which is not always practical.
Advanced Methods: Mathematical models of the battery's behavior are used in more complex methods for SOC estimation. The Extended Kalman Filter (EKF), which may integrate a model of the battery with readings of voltage and current to estimate SOC, is one of these techniques. Neural networks are a more sophisticated technique that uses machine learning to train a model to estimate SOC from historical data. Although more computationally complex, more sophisticated algorithms can deliver SOC estimates that are more accurate.
In order to estimate SOC precisely and dependably, many battery management systems combine a number of these techniques. Considering the needed precision, the nature of the application, and the available computational resources can help you choose the best approach or combination of methods for a given application.
State Of Health (SOH)
Definition and Importance
Status of Health (SOH) is a metric used to compare a battery's current status to that of a brand-new battery. SOH is measured as a percentage, where 100% corresponds to a brand-new battery in ideal condition and lower values to deterioration and aging.
For a number of reasons, it's crucial to understand a battery's SOH:
Performance Assessment: SOH sheds light on a battery's present performance potential. In applications where battery performance is essential, such as electric automobiles or backup power sources, it is essential.
Maintenance and Replacement Decisions: Users may avoid unexpected failures and reduce expenses by monitoring SOH and making educated decisions about whether to perform maintenance or replace a battery.
Safety: The safety of a battery with a drastically reduced SOH may have been jeopardized. For instance, degeneration in lithium-ion batteries may result in internal short circuits brought on by dendritic growth within individual cells, which may trigger an uncontrollable heat event. A low SOH will also increase the battery's internal resistance, which will result in higher heat dissipation inside the battery during use.
Factors affecting SOH
The condition of a battery can be impacted by a number of variables, including:
Cycling: The battery undergoes chemical changes as a result of repeated charging and discharging cycles, which over time produce a reduction in capacity and, ultimately, SOH. For batteries, this is a normal, inescapable occurrence.
Temperature: Temperature has an impact on batteries. Temperatures can hasten damage at both high and low levels. For instance, lead-acid batteries' electrolyte may evaporate at high temperatures. The most susceptible to low temperatures are Li-ion batteries. The battery's capacity will degrade over time, but repeated exposure to low temperatures might hasten this process. It is crucial to utilize the correct battery type for the specified temperature range, or to employ a mechanism to regulate the temperature, such as a fan or liquid cooling system.
Depth of Discharge (DoD): Regularly deep draining a battery to low levels of charge can hasten deterioration. The BMS systems prevent a battery cell from being discharged below a specific limit voltage.
Overcharging: Overcharging a battery can result in harmful chemical reactions that damage the battery. Additionally, it can increase in pressure, resulting in outgassing or battery enclosure damage. The BMS systems also prevent batteries from being overcharged.
Current Magnitude: High currents might speed up deterioration and cause localized heating when charging or discharging. The BMS system's responsibility also includes maintaining a current limit.
Storage Conditions: A battery's SOH can be impacted by how and where it is kept, particularly during extended periods of inactivity. Batteries must be kept in a cool, dry area with an ambient temperature of between 15°C and 25°C for the majority of battery chemistries in order to retain a decent SOH for an extended period of time. Moisture should also be kept to a minimum since it can harm the connections and increase their susceptibility to corrosion, which can cause the battery to overheat when used.
Estimation Methods
Calculating a battery's SOH requires intricate analysis of several traits and attributes. Following are some popular techniques for SOH estimation:
Direct Measurement: This entails tracking alterations in physical parameters that are related to battery health, such as capacity or internal resistance. For instance, a battery's SOH may be indicated by a gradual decline in its maximum charge capacity.
Model-Based Methods: In these methods, the fundamental electrochemical processes in the battery are represented mathematically. The SOH may be calculated from the model parameters by making adjustments depending on data from the battery.
Data-Driven Methods: SOH may be calculated using statistical and machine learning techniques using past data. A model can learn to estimate SOH for a certain set of metrics by being trained with data from batteries that are degrading.
Hybrid Methods: To estimate SOH, these techniques incorporate elements of data-driven, model-based, and direct measurement techniques. This could entail combining a machine learning algorithm and a mathematical model, for example.
SOH estimate is a difficult problem in reality but is essential for the safe and dependable functioning of batteries, especially in high-performance applications. In-depth algorithms and models are used by advanced battery management systems to continually monitor and assess the condition of health of batteries in real-time.
Other Parameters
Nominal Voltage
The standard operating voltage of a battery is indicated by a reference value known as nominal voltage. It is a standardized measurement that illustrates the voltage range in which a battery typically functions. A normal alkaline cell, for instance, has a nominal voltage of 1.5 volts, while a typical lithium-ion cell has a nominal voltage of 3.7 volts. It is crucial to understand that a battery's nominal voltage is used to classify and compare batteries, whereas the actual voltage of a battery changes during the course of its discharge cycle. The following image shows a typical discharge curve for both lead-acid and lithium-ion batteries:
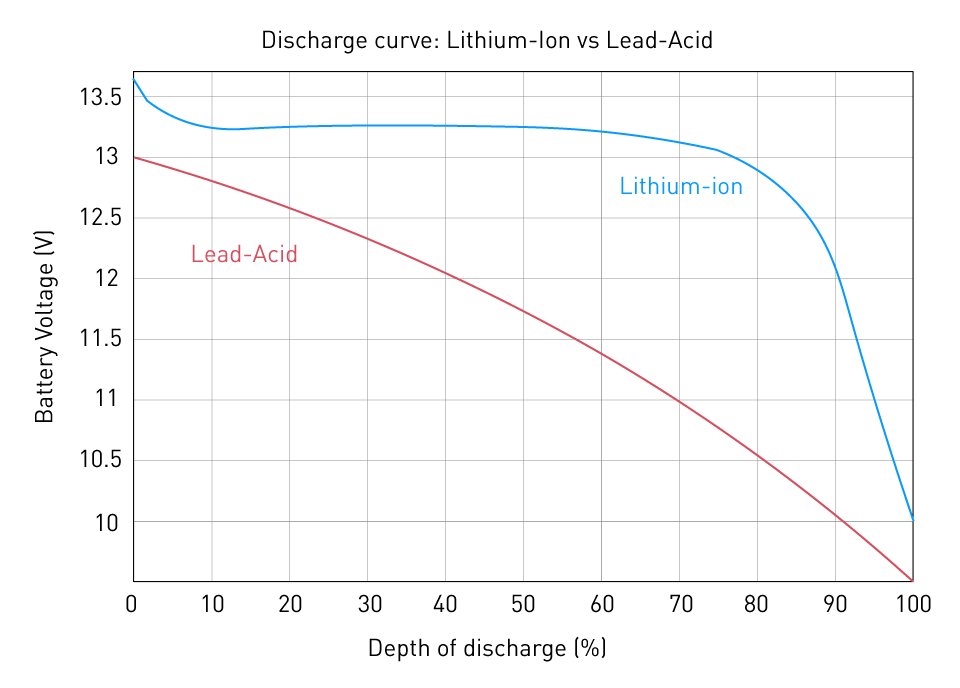
Figure 7: Discharge curve comparison of Lithium-ion and Lead-Acid battery
As we can see, a lithium-ion battery tends to maintain a constant output voltage throughout its discharge, but a lead-acid battery loses voltage practically linearly and more quickly.
Internal Resistance
A battery's internal resistance is the resistance to the passage of electric current inside the battery. The ionic resistance in the electrolyte, the inherent resistance of the materials, and contact resistance at component interfaces are a few of the causes. Several factors, including the internal resistance, can impact the battery's performance:
Voltage Drop: The voltage drop inside the battery during discharge is greater with a higher internal resistance, which lowers the voltage available across the battery terminals.
Heat Generation: Battery heat is produced when current passes through the internal resistance. The health and longevity of the battery might be harmed by too much heat.
Reduction in Efficiency: Due to internal resistance, energy is wasted as heat, lowering the battery's total energy efficiency.
Cycle Life
Cycle Life, a gauge of a rechargeable battery's endurance, is the number of full charge and discharge cycles a battery can go through before losing any of its capacity (usually 80% of its initial capacity). A battery with a 1000 cycle rating, for instance, may be completely charged and drained 1000 times before losing 80% of its initial capacity.
The depth of discharge, charging rate, temperature, and material qualities of the battery are some of the variables that affect cycle life. It is a crucial variable, particularly in applications like electric cars and energy storage systems where long-term dependability and a low total cost of ownership are crucial.
Self-Discharge Rate
Even when there isn't a load attached to a battery, it is still losing charge. It's known as self-discharge. Every battery chemistry has a unique self-discharge rate, though. The ambient temperature has a significant impact on the self-discharge rate since it causes it to rise as the temperature rises. The self-discharge rate for various main and secondary batteries is displayed in the following table:
Table 1: Self-discharge rate comparison of various batteries
| Battery Chemistry | Rechargeable | Typical self-discharge or shelf life |
| Lithium metal | No | 10 years shelf life |
| Alkaline | No | 5 years shelf life |
| Zinc-carbon | No | 2-3 years shelf life |
| Lithium-ion | Yes | 2-3% per month |
| Lithium-polymer | Yes | ~5% per month |
| Low self-discharge NiMH | Yes | As low as 0.25% per month |
| Lead-acid | Yes | 4-6% per month |
| Nickel-cadmium | Yes | 15-20% per month |
| Nickel-metal hydride (NiMH) | Yes | 30% per month |
Instead of self-discharge rate, we utilize the idea of shelf life for main (non-rechargeable) batteries. The self-discharge rate is a crucial factor in some delicate applications. For instance, if the batteries may be idle for a long time in aircraft and satellite applications, medical devices like peacemakers, etc.





直接登录
创建新帐号