Basic Construction of Starter Batteries
Lead-Acid Batteries
Lead-acid batteries, the most traditional form of rechargeable batteries, have served as the cornerstone of automotive starting systems for over a century. The construction of these batteries is straightforward yet strong:
Plates: Within these batteries lie a sequence of positive plates coated in lead dioxide and negative plates crafted from sponge lead. In an electrolyte solution, these plates are submerged.
Electrolyte: The electrolyte, a solution of sulfuric acid (H2SO4), aids in the movement of ions between the plates when the battery undergoes charging and discharging cycles.
Separators: Between the positive and negative plates, thin sheets composed of plastic or similar insulating material are positioned to deter short circuits.
Case: In a durable plastic case that is resistant to high temperatures and acid, the complete assembly is placed.
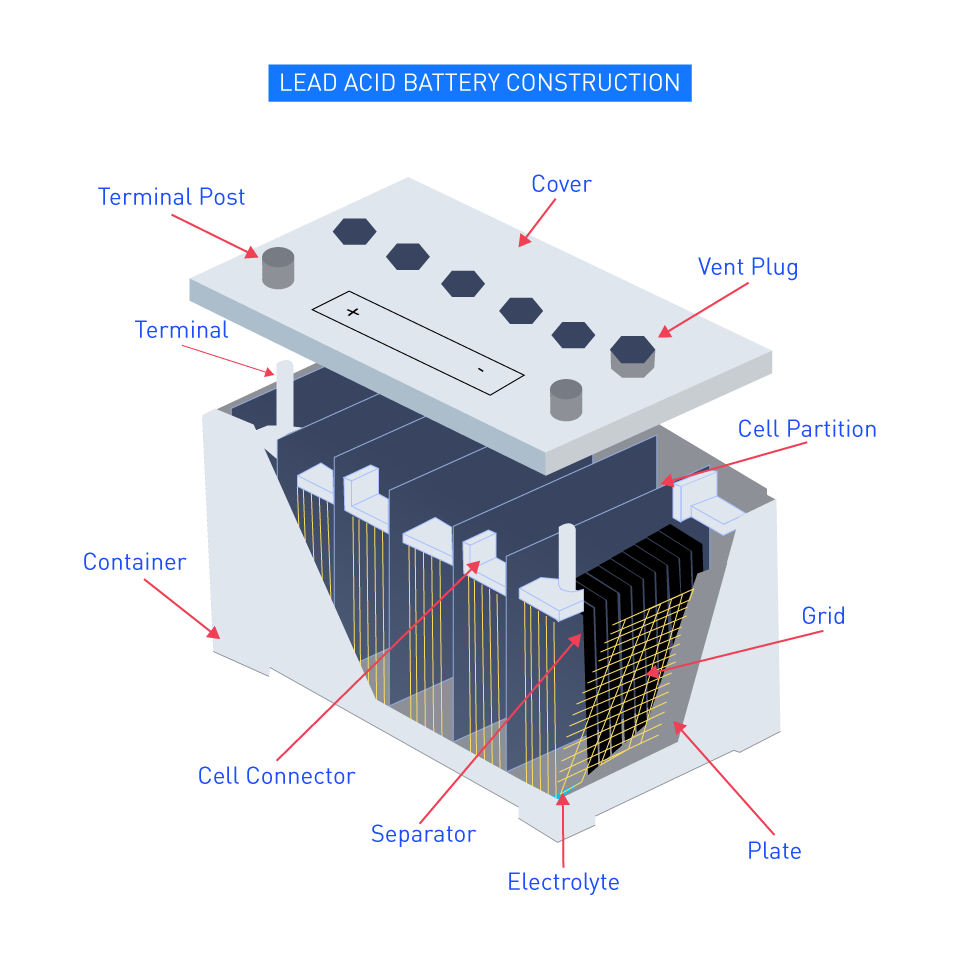
Figure 1: Construction of Lead-Acid Battery
Absorbent Glass Mat (AGM) Batteries
A subtype of lead-acid batteries is known as AGM batteries, that provides multiple advancements over traditional flooded designs:
Mat Construction: The primary distinction lies in incorporating thin glass fibers interlaced into a mat. Eradicating the unrestricted liquid found in conventional lead-acid batteries, this specialized mat is engineered to soak up and immobilize the electrolyte.
Compression: Compressing the plates in AGM batteries is a common practice known to enhance both the battery's lifespan and its overall performance.
Benefits: AGM batteries boast a construction that minimizes the likelihood of leakage, enables flexible mounting positions, and enhances resistance to vibrations. Additionally, unlike flooded batteries, they usually exhibit a reduced self-discharge rate and possess the capability to deliver higher currents.
Enhanced Flooded Batteries (EFB)
The batteries that bridge the gap between traditional flooded lead-acid batteries and AGM batteries are enhanced flooded batteries:
Polyester Scrim: Introducing a polyester scrim (a thin layer) amidst the plates, EFB batteries enhance the battery's durability and performance by preserving active material that would otherwise dissipate during the cycling process.
Dual-Plate Separation: Combining traditional separators with the mentioned polyester scrim, EFBs commonly showcase enhanced charge acceptance and increased cycle durability.
Applications: EFB batteries are experiencing a surge in popularity among vehicles equipped with start-stop systems as a result of their equilibrium between performance and cost-effectiveness. As compared to traditional ignition systems, these systems demand more from a starter battery.
Figure 2: Construction of EFB Battery
To summarize, although these three battery types can work based on lead-acid chemistry principles, their construction diverges to fulfil performance, cost objectives, and durability. Determining the most suitable option frequently relies on the particular requirements of the vehicle and its intended application. This can range from standard starting duties in typical cars to the increased demands found in start-stop systems and luxury vehicles.
Chemical Reactions and Energy Storage
Electrochemical Reactions in Lead-Acid Batteries
Electrochemical reactions are a coordinated way of ionic movement and electron transfer, and it is present at the center of the lead-acid battery’s ability to keep and release energy. A fundamental aspect lies in comprehending these reactions to grasp the functionality of these batteries. This understanding elucidates why they have endured as a cornerstone in automotive applications for an extended period.
Discharge Reaction (Battery Providing Power)
When the battery supplies power to a load (e.g., starting an engine) during discharge, the following reactions occur:
At the Positive Plate
$$PbO_2+3H_2SO_4\rightarrow PbSO_4+2H_2O+2H^++2e^-$$Releasing hydrogen ions (H⁺) and electrons (e⁻), lead dioxide (PbO₂) is reduced to lead sulfate (PbSO₄).
At the Negative Plate
$$Pb+H_2SO_4\rightarrow PbSO_4+2H^++2e^-$$While releasing hydrogen ions and electrons, lead (Pb) reacts with sulfuric acid (H₂SO₄) to form lead sulfate (PbSO₄).
Before re-entering the battery at the positive plate, these electrons flow through the external circuit, offering electric power and facilitating the aforementioned reactions.
Charge Reaction (Battery Being Recharged)
The reactions are reversed when recharging:
At the Positive Plate
$$PbSO_4+2H_2O\rightarrow PbO_2+3H_2SO_4+2e^-$$Consuming electrons from the external circuit, lead sulfate and water are oxidized to regenerate lead dioxide and sulfuric acid.
At the Negative Plate
$$PbSO_4+2e^-\rightarrow Pb+H_2SO_4$$Using the provided electrons, lead sulfate is reduced to form lead.
Key Takeaways and Implications
- Lead Sulfate Formation: During discharge, a notable characteristic involves the creation of lead sulfate on both plates, marking a significant aspect of these reactions. When a battery remains in a discharged state for prolonged durations, the lead sulfate can crystallize, resulting in diminished capacity and the potential for battery failure. This occurrence, termed sulfation, is a well-known phenomenon in battery degradation.
- Water Consumption: It's important to note the utilization of water in the discharge reaction occurring at the positive plate, which is subsequently replenished during the charging process. Excessive water consumption can be caused by overcharging of a lead-acid battery, potentially causing a reduction in electrolyte levels and subsequent damage to the battery.
- State-of-Charge (SOC) Indicator: For the battery's charge status, the density or specific gravity of the sulfuric acid solution acts as an indicator. A lower density indicates discharge while a higher density signals a more charged battery.
In conclusion, the longevity and functionality of the lead-acid battery are directly tied to these electrochemical reactions. For optimal battery performance, proper maintenance is paramount, which ensures these reactions proceed efficiently.
Factors Affecting Battery Health
For battery’s optimal functioning, safety, and longevity, battery health is imperative. The health of starter batteries in vehicles is adversely impacted by numerous factors, reducing their capacity and complete lifespan. The major factors that affect battery health are as follows:
Temperature Extremes
- Effect: Temperature changes significantly impact the performance of batteries. The battery’s internal resistance can be elevated by cold condition, hampering its power delivery. On the other hand, elevated temperatures accelerate internal chemical reactions, increasing the risk of overcharging, depletion of water, and hastened degradation.
- Mitigation: Incorporating thermal management systems is a common practice in modern vehicles. These systems serve to maintain the battery within its ideal temperature range, thereby extending both its lifespan and performance capabilities.
Overcharging and Deep Discharge
- Effect: A significant deterioration can be caused by overcharging or deeply discharging a battery. Excessive charging generates surplus heat and gas inside the battery, potentially leading to warping of internal plates or, in extreme cases, triggering an explosion. Sulfation, a consequence of deep discharges, occurs when sulfate crystals develop on the battery's lead plates, resulting in a subsequent reduction in its capacity.
- Mitigation: Usually found in contemporary vehicles, advanced charging systems are equipped with current and voltage regulators to avert overcharging. Moreover, to prevent deep discharges, Battery Management Systems (BMS) monitor the state of charge.
Vibration and Mechanical Stresses
- Effect: Caused by vehicle vibrations or impact, mechanical stresses can result in internal short circuits or hamper the battery’s casing.
- Mitigation: Specialized compartments in vehicles typically house batteries, providing safeguarding against mechanical shocks. To avert vibrations, damping materials might also be employed.
Age and Cycles
- Effect: A battery’s capacity naturally diminishes with repeated charging and discharging cycles over time. In the battery's internal chemistry, the decline occurs due to gradual alterations and the cumulative effects of physical wear and tear.
- Mitigation: Adhering to the manufacturer's guidelines and utilizing a battery within its designated operating parameters can aid in maximizing its lifespan, despite the inevitable ageing process.
Poor Maintenance
- Effect: Failure to perform maintenance tasks, like neglecting to refill distilled water in specific battery variants or permitting terminal corrosion to occur, can undermine the battery’s performance.
- Mitigation: Sustaining battery health involves adhering to regular inspection schedules and maintenance routines, such as verifying adequate fluid levels and cleaning terminals.
Through diligent care, consistent monitoring, and effective mitigation strategies, the longevity and performance of a starter battery can be maximized, despite numerous influencing factors on its health.
Maintenance and Safety Considerations
Despite their durable design and long lifespan, automotive batteries need regular maintenance and careful handling to guarantee both optimal performance and safety. Given their vital function in initiating a vehicle's engine, the maintenance and safety considerations for starter batteries become increasingly pivotal within this context. Within this segment, there is an exploration of the preventive maintenance methods and the essential safety measures required when handling starter batteries.
Preventive Maintenance Techniques
The life of a starter battery can be extended by proper maintenance, ensuring that it works optimally throughout its complete service life.
Regular Inspection: Any signs of damage to the battery casing, corrosion on the terminals, or any leakage can be identified by scheduled periodic checks. Potential battery issues can be identified by these early indicators.
Terminal Cleaning: Terminals can accumulate corrosion over time, which can affect the current flow. Good electrical contact can be maintained by cleaning terminals, with a mixture of baking soda and water, followed by a complete rinse with clean water.
Maintain Electrolyte Level: It is imperative to make sure that the electrolyte level must be between the minimum and maximum marks for flooded lead-acid batteries. If the level drops, distilled water should be used.
Charge State Monitoring: Reduced life and performance can be experienced by an undercharged or overcharged battery. Use of a hydrometer or voltmeter can be advantageous for periodic testing of the battery’s state of charge.
Secure Mounting: To prevent potential internal damage or short circuits, it's important to securely mount the battery to avoid excessive vibration.
Jump-Starting the Battery
Using jumper cables, jump-starting involves connecting a vehicle with a drained battery to another vehicle's fully charged battery as a technique to initiate the former. When properly executed, this process permits the vehicle with the depleted battery to access the required power to start its engine. Nevertheless, exercising caution is crucial.
Procedure: Start by placing the vehicles in close proximity, ensuring there's enough distance for the jumper cables to connect while also making sure they do not come into contact with each other. Before proceeding, make sure that both vehicles are turned off. Begin by attaching the positive (usually red) jumper cable to the positive terminal of the dead battery. Then, connect the opposite end of the cable to the positive terminal of the functional battery. Following that, link the negative (commonly black) cable to the negative terminal of the functioning battery. Finally, attach the opposite end of the negative cable to an unpainted metal surface on the disabled vehicle, away from its battery. This acts as a grounding point, ensuring a proper connection.
Start the Good Vehicle: Activate the vehicle's engine equipped with the charged battery to enable its alternator to generate electricity. Try starting the car with the dead battery after waiting a few minutes.
Disconnecting: Beginning with the negative grounding connection, disconnect the jumper cables in the reverse order of connection once the vehicle is started.
Potential Risks: Mishandling the jump-start process carries various risks. Incorrectly connecting the cables or bridging the terminals can result in a short circuit, potentially causing sparks or, in extreme cases, an explosion. Moreover, in modern vehicles, a sudden surge of electricity can harm the electronics. Prior to initiating a jump-start, it is advisable to consult the vehicle's manual since certain vehicles may contain specific instructions or precautions that should be taken into account.
Reviving a dead battery through jump-starting is beneficial, but it's essential to adhere to the correct procedures and maintain caution consistently.
Safety Precautions and Handling
Due to the chemicals involved and the risk of electrical shock, operating batteries need a keen sense of protection.
Wear Protective Gear: Make sure to wear protective gloves and safety glasses to protect from acid splashes or spills whenever handling batteries or doing maintenance work.
Ventilation: Flammable hydrogen gas can be emitted by batteries especially in case of charging. To avoid gas accumulation, make sure to work in well-ventilated areas.
Avoid Flames and Sparks: It is imperative to keep batteries away from sparks, open flames, or any potential sources of ignition due to the risk of gas emission.
Handle with Care: Specially lead-acid types of batteries can be heavy. To avert injury and make sure that the battery is placed securely once lifted, use appropriate lifting techniques.
First Aid: Rinse immediately with a lot of water and seek medical attention whenever acid comes in contact with the skin or eyes. It is advantageous to have a first aid kit and an eye-wash station nearby.
Starter batteries, although engineered for durability, heavily rely on maintenance practices and handling methods to sustain their health and longevity. Prioritizing preventive care and safety through a proactive approach guarantees the consistent and reliable long-term performance of these batteries.
Lithium-Ion Batteries as Starter Batteries
Despite gaining widespread popularity in diverse applications like electric vehicles (EVs) and consumer electronics, Lithium-ion (Li-ion) batteries haven't found extensive use as starter batteries in conventional combustion engine vehicles. This choice is underpinned by multiple reasons:
Cold-Cranking Amperage (CCA): Often employed as starter batteries, traditional lead-acid batteries possess a notable Cold Cranking Amperage (CCA) value, supplying the requisite high current for brief periods to initiate a combustion engine, particularly in colder environments. Despite their ability to provide high currents, the performance of Li-ion batteries experiences substantial degradation in colder temperatures.
Cost Considerations: In terms of initial investment, Li-ion batteries are more costly than traditional lead-acid batteries. Due to their primary function of starting the engine in combustion vehicles, the potential benefits of Li-ion batteries might not justify the additional cost for this specific application.
Lifecycle Compatibility: The design of combustion engine vehicles necessitates only a brief burst of energy to start the engine, thus avoiding deep discharge of their starter batteries. The intended design of Li-ion batteries involves accommodating deeper discharge cycles, but their lifespan can be negatively impacted by frequent shallow discharges.
Safety Concerns: Lead-acid batteries, when damaged or mismanaged, exhibit less volatility compared to Li-ion batteries despite the latter's higher energy density. Li-ion batteries pose a risk of thermal runaway and fire in case of an accident, which can complicate the implementation of safety measures.
Integrated Battery Management System (BMS): Monitoring and balancing cell voltages, managing the state of charge, and maintaining safe operating conditions necessitate a sophisticated Battery Management System (BMS) for Li-ion batteries. Complexity and cost can be added as a BMS for a starter battery application.
Overcharge Tolerance: Compared to Li-ion batteries, lead-acid batteries typically exhibit greater tolerance towards overcharging. In certain automotive situations, overcharging conditions can lead to rapid degradation of the latter (referring to Li-ion batteries).









直接登录
创建新帐号